The GI Dynamics company has suspended the CE marking from its endoscopic device for diabetes, marketed under the name of Endobarrier.
According to information published by Fiercebiotech, the cancellation has occurred due to its quality management system, making it clear that "this action does not question the security and effectiveness of Endobarrier and does not involve its withdrawal."
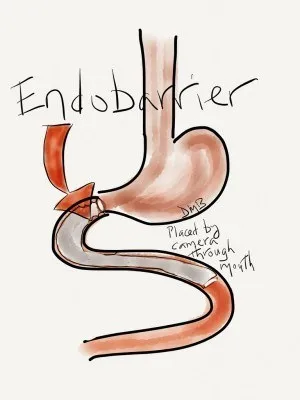
The device is designed for people whose diabetes medications no longer work and are at risk of complications, or even obesity.Specifically, it is a flexible tube -shaped lining that forms a shield between food and part of the intestinal wall.
"This action does not question the security and effectiveness of Endobarrier and does not involve its withdrawal," said Scott Schorer, CEO of Gi Dynamics."We are working to address the problems of our quality management system to restore CE marking as soon as possible," said the manager.
On the other hand, from the company they have indicated that all patients implanted with an endobarrier can continue their treatment under the supervision and evaluation of the health professional that takes their case.